Mesut Sahin
In medical sciences, among many other subjects, we learn about human body parts (anatomy), how the body functions at the cellular (biology) and systemic levels (physiology), its diseases (pathology), and the symptomatic treatment of these diseases through drug therapy (pharmacology). In general the approach taken by medicine is to understand the natural functions of the body in a balanced state (homeostasis) and to try to restore this balance when it is upset by a disease or an invading force. In engineering disciplines, however, the approach taken towards nature is completely different. We study nature, understand the mathematical principles that govern its operations, and use this knowledge to build new systems. The term “engineering” is synonymous with the concept of “designing” new things using human experience and intelligence.
The discipline in which medicine and engineering truly meet and face new challenges is the field of “biomedical engineering,” an emerging discipline that is only a few decades old. In each sub-specialty of biomedical engineering, researchers study the human body, develop new materials and structures using engineering sciences, either as a treatment method for disease (e.g. artificial bone implants, artificial blood, vascular stents, cardiac valves, etc.) or to diagnose them (e.g. imaging methods and other diagnostic instruments in hospitals). Biomedical engineers face the incredible challenge of developing materials and devices that are compatible with biological systems and capable of working inside the human body to substitute bodily functions. Needless to say, the extreme complexity of the human body makes it impossible to mimic the original system or function of the organs in any way. However, even a poor replacement part or a functional improvement provides great benefit to the patients.
One of the most complex systems of the human body is the nervous system, which consists of the central area (the brain and the spinal cord) and the peripheral parts. The branch of biomedical engineering that deals with the nervous system is “neural engineering.” In this article, we will touch upon a specific subject in the broader area of neural engineering, that is, “neural prosthetics.”1 As the name implies, neural prosthetics is an area where engineering knowledge is utilized to treat neural disorders.
The building blocks of the nervous system are called “neurons.” Neurons generate electric pulses to communicate with each other. The fact that these electric pulses can be elicited by artificial means, i.e. by applying small electric currents to the neurons externally, forms the very foundation of the field of neural prosthetics. Neural engineers can input information into the nervous system by taking advantage of this phenomenon, called “neural stimulation.” Likewise, the information content of neuronal activity can be deciphered by recording the electrical pulses from the neurons and interpreting them according to neuronal function. This two way traffic, monitoring and controlling the neural activity, allows researchers in this field to develop methods of treatment for some sensory, motor, and psychological disorders.
Some of the most successful neural prosthetic applications have been in deep brain stimulation in Parkinson’s disease,
|
Figure 1: Components of a cochlear implant by Advanced Bionics Corp. (www.bionicear.com). A: The sound processing unit including a microphone, B: the transmitting antenna, C: the implant, which sends the electric signals down to the electrode array through tiny wires, D: the electrode array stimulates the hearing nerve in the inner ear, which carries the sound information to the brain to be heard.
|
cochlear prosthesis in hearing impairment, bladder emptying and respiration in spinal cord injury, and vagus nerve (10th cranial nerve) stimulation in epilepsy and psychological depression. These are neural prostheses that are readily available as a treatment method for the given ailments. There is a whole host of others that are in the research and development phase. We will review a couple of examples.
In certain diseases of the inner ear hearing is lost as a result of damage to the hair cells inside the cochlea. In normal cochlea the sound information reaches these hair cells after traveling through the ear drum (tympanic membrane) and the structures of the middle ear, causing them to vibrate. This vibration of the hair cells is mechanically transported to the spiral ganglion cells that form the hearing (auditory) nerve. The hearing nerve carries the sound information to the brain in the form of electric pulses. The ganglion cells are healthy and functional even if the entire population of hair cells has been lost as a result of disease. Neural engineers take advantage of the fact that the spiral ganglion cells (which normally accept input from the hair cells) can be electrically stimulated, thus mimicking the function of the hair cells and producing the sensation of sound.2 During a simple surgical operation, the surgeon inserts an electrode into the ear canal which spirals into the lumen of the cochlea so that the sites where the electric current emits from the electrode are adjacent to the spiral ganglion cells (Figure 1). To summarize the principle of the operation; the audio signals are captured by a microphone, processed, converted into electric pulses (A in Figure 1), and transmitted to the implant over a transmitting antenna (B in Figure 1), or headpiece, held in place by magnets. The implant (C in Figure 1) applies the signals to the ganglion cells in the cochlea through tiny electrodes (D in Figure 1). The hearing nerve (auditory nerve) carries the sound information to the brain, where it is “heard.”
|
Figure 2: Intraocular epiretinal prosthesis conccept. An external video camera would capture an image and a custom microelectronic unit would process the image and transmit data and power to the implant via radio frequency communication. The implant would receive data and power and stimulate the retina with the command pulse pattern (adapted from Weiland and Humayun see note 8).
|
Even though the human spiral ganglion has tens of thousands of nerve cells that provide a rich sense of hearing, the cochlear implant, using only six stimulation contacts, can produce auditory perception with sufficient fidelity to enable a deaf individual to use an ordinary telephone.3 Individuals with cochlear implants can also improve their hearing with practice. Thousands of patients have been implanted with cochlear prostheses to date, including children.
The second neural prosthesis application we will review is the retinal prosthesis, which, unlike the cochlear implants, is still in the research phase. Retinitis pigmentosa and age-related macular degeneration both lead to photoreceptor degeneration in the eye and result in a significant visual deficit or blindness.4 A growing body of research supports the feasibility of replacing the function of the photoreceptors with an electronic device. 5–7 A retinal prosthesis is analogous to the cochlear implant in many ways. In a healthy retina, the photoreceptors initiate a neural signal in response to light. In a retinal prosthesis, electrical pulses are utilized to initiate a neural response in the remaining cells of the retina, the bipolar and ganglion cells. It is hypothesized that the perception of shapes and images will be possible through pattern stimulation of the retina. Initial results are encouraging, but the quality of vision that can be attained with this approach is still a question to be answered. A conceptual retinal prosthesis system is shown in Figure 2. The system consists of an external unit coupled to an implanted stimulator with a wireless link. A video camera in the external unit captures an image and converts it to digital data. The implanted unit receives the signal, recovers power and data from the signal, and generates the stimulating current. The stimulus pattern is applied to the retina via the electrode array, which contains distinct electrodes that interface at many locations on the retinal surface. Recent implants in human subjects suggest the feasibility of this approach where individuals attain perception of bright dots in the visual field called 'phosphenes.' Furthermore, blind subjects are able to perceive edges when a few of these bright dots are lined up in their visual field.
 |
|
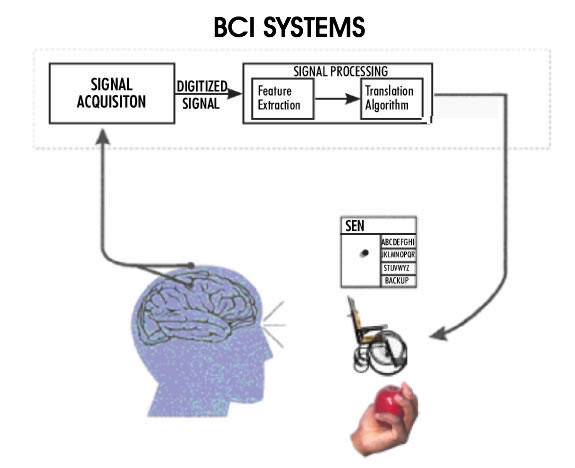
Figure 3: A conceptual diagram of a Brain-Computer Interface for high level spinal cord injury or patients with 'locked-in syndrome' (adapted from Wolpaw et al., see note 9). The recorded neural activity from the motor cortex is processed and converted into command signals to control, for instance, a wheelchair, or to generate electrical signals to activate hand muscles for grasping an object.
|
Both cochlear and retinal implants are sensory prostheses, i.e. aids for sensory impairments. Another family of neural prostheses deals with motor impairments. Severe motor disability results from high level spinal cord injuries (quadriplegia) where most of the body is paralyzed, sparing only some face, neck, and shoulder muscles. Quadriplegic individuals are in extreme need of a means to control their environment; they need to be in control of their wheelchairs, bed, the room temperature, lights, TV, etc. Because of the level of paralysis it is impossible for them to generate any control signal, except perhaps by sipping or puffing on the end of a tube, which produces a very poor control signal. In the case of a 'locked-in syndrome' the condition of the patient is even more serious, with only some functions remaining in the facial muscles. The term 'brain-computer interface' has been coined to refer to attempts whereby the motor output of the brain is recorded and interpreted to generate the control signals needed by these patients (Figure 3,9). The ultimate objective of this research will be accomplished when the patients are able to control anything they need to control in their environment, including a computer. The brain-computer interfaces vary in the invasiveness of the approach. The least invasive methods utilize the electroencephalogram (EEG) signals recorded from the scalp. Unfortunately, the signal quality is poor and only 'on/off' type of command signals can be generated using this method. In the most invasive, yet most successful applications, an array of electrodes is implanted directly into the motor cortex of the brain at a depth of a couple of millimeters. The recorded signals contain volitional information as the patient makes intentions to move their arms or legs. These signals can be controlled by the patient, and they can in turn be used to control their environment. The current level of success in this type of BCI allows the user to have three dimensional control of a robot arm. This is of invaluable benefit to a quadriplegic individual.
Concluding Remarks: Reflections on Divine Wisdom
Even the subtlest parts of the nervous system are extremely complex. Just to name a few examples, from the highest centers in the brain down to the skeletal muscles in a descending order; the neural circuits of the short-term memory in the hippocampus, fine motor control circuits of the cerebellum, central pattern generators in the spinal cord, and even the control of skeletal muscles in graceful movements of the limbs are impossible to reproduce by artificial means. The Seal of Divine Design is clearly visible in these neural systems, as they are far more complex, far more compact, and far more functionally efficient than any system engineered by mankind. If anything, the growing experience in neurosciences teaches us that the vertebrate nervous system is full of wonders of engineering design. Therefore, it is a great blessing to be a student of both neurosciences and engineering disciplines. This bestows neural engineers with a unique perspective to understand the beauty embroidered into the human nervous system and contemplate on the Divine Wisdom. In spiritual terms, we may think of the human nervous system as a window opening to the works of Divine Wisdom, with manifestations of His Beautiful Names at the brightest level. It is an overwhelming joy to be able to open this window a crack, once in a while, and take a little peek.
References
- Wise, K.D. 'Silicon microsystems for neuroscience and neural prostheses,' IEEE Engineering in Medicine and Biology Society Magazine, vol. 25, no. 5, pp. 22- 29, Sept.-Oct., 2005.
- G.E. Loeb, 'Cochlear prosthetics,' Annu. Rev. Neurosci., vol. 13, pp. 357–371, 1990.
- J. Helms, V. Weichbold, U. Baumann, H. von Specht, F. Schon, J. Muller, B. Esser, M. Ziese, I. Anderson, and P. D"Haese, 'Analysis of ceiling effects occurring with speech recognition tests in adult cochlear-implanted patients,' ORL J. Otorhinolaryngol Relat. Spec., vol. 66, no. 3, pp. 130–135, 2004.
- E.L. Berson, 'Retinitis pigmentosa. The friedenwald lecture,' Invest Ophthalmol. Vis .Sci., vol. 34, no. 5, pp. 1659–1676, Apr. 1993.
- E. Zrenner, 'Will retinal implants restore vision?,' Science, vol. 295, no. 5557, pp. 1022–1025, Feb. 2002.
- J.F. Rizzo III, J. Wyatt, J. Lowenstein, S. Kelly, and D. Shire, 'Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short term surgical trials,' Invest. Ophthalmol. Vis. Sci., vol. 44, no. 12, pp. 5362–5369, 2003.
- M.S. Humayun, J. Weiland, G. Fujii, R.J. Greenberg, R. Williamson , J. Little, B. Mech, V. Cimmarusti, G. van Boemel, G. Dagnelie, and E. de Juan, Jr., 'Visual perception in a blind subject with a chronic microelectronic retinal prosthesis,' Vision Res., vol. 43, no. 24, pp. 2573–2581, 2003.
- Weiland, J.D. and Humayun, M.S., 'A biomimetric retinal stimulation array,' IEEE Engineering in Medicine and Biology Society Magazine, vol. 25, no. 5, pp. 14-21, Sept.-Oct., 2005.
- Wolpaw J.R. et al., 'Brain-computer interfaces for communication and control,' Clinical Neurophysiolology, vol. 113(6), pp. 767-791, 2002.